Types and characteristics of killed bacteria as a feed additives
2020년 07월 21일
- Nutrition
SUMMARY
✔Currently, Escherichia coli (E. coli) and Corynebacterium (Coryne.) are mainly used as amino acid industrial fermentation strains for food or feed additives. These are classified into Gram-negative and Gram-positive bacteria, respectively.
✔The biggest difference between Gram-negative bacteria and Gram-positive bacteria is the presence or absence of Lipopolysaccharide (LPS), a component of the cell wall (Gram-negative bacteria have LPS, but Gram-positive bacteria do not have LPS).
✔LPS is an intracellular toxin (endotoxin), which is not affected when consumed under healthy situations, but maybe harmful to health if it enters the blood due to internal organ wounds.
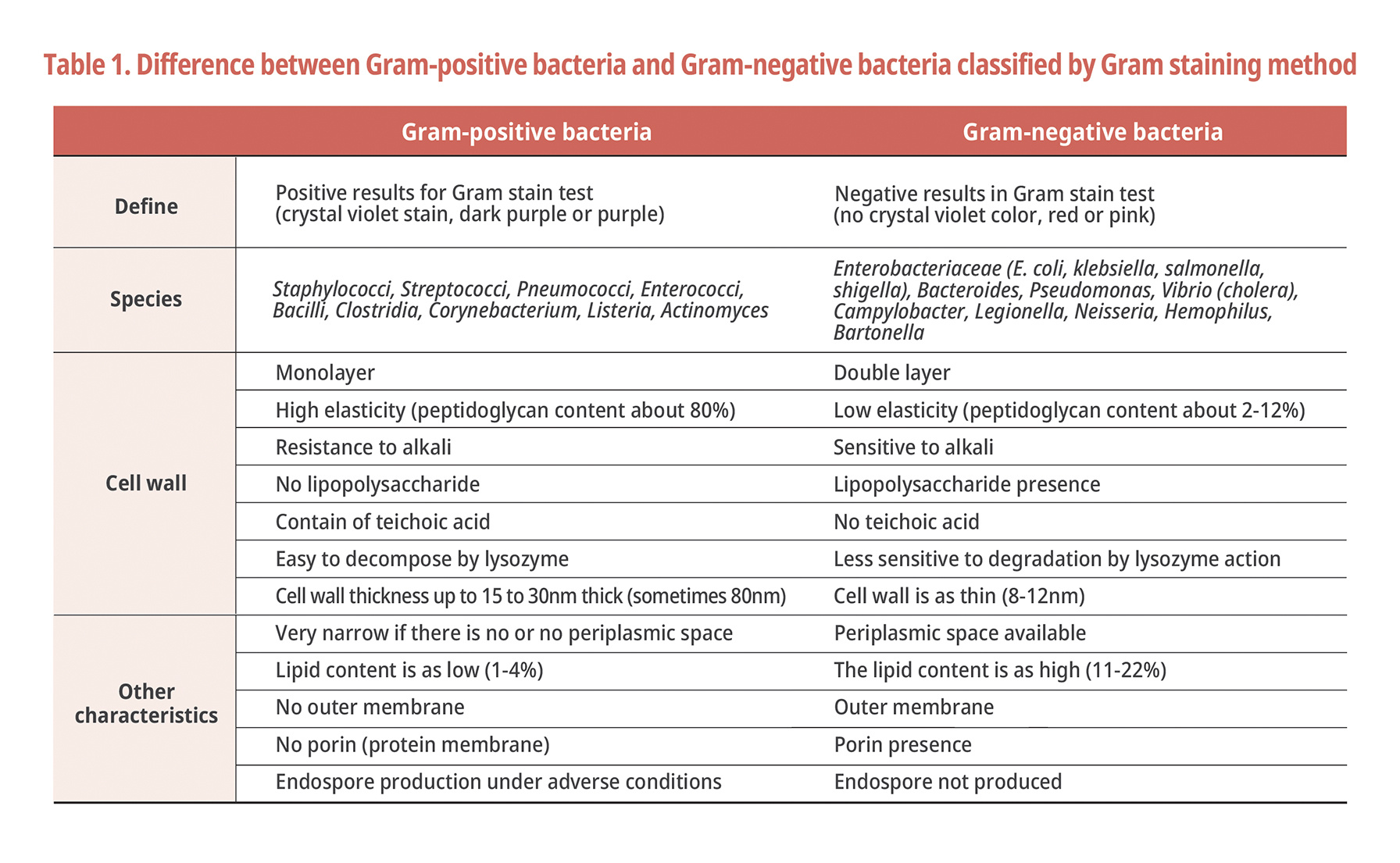
Difference between Gram-negative bacteria and Gram-positive bacteria
Strains are largely classified by morphology, nutrition, flagella number, and cell wall [1]. Among various classification methods, a bacterial identification method by gram's stain, which can find a suitable strain for cultivation or sterilization of bacterial species, is commonly used [2]. The Gram staining method developed by Danish scientist Hans Christian Gram in 1884 is a very important staining method that defines the characteristics of bacteria because the staining result varies depending on the cell wall component (structural difference of the cell wall) that determines the molecular pattern derived from microorganisms. Even now, it is very important and useful tool in the research of bacteria. Introducing the classification criteria by Gram staining method and the differences are detailed in Table 1 below.
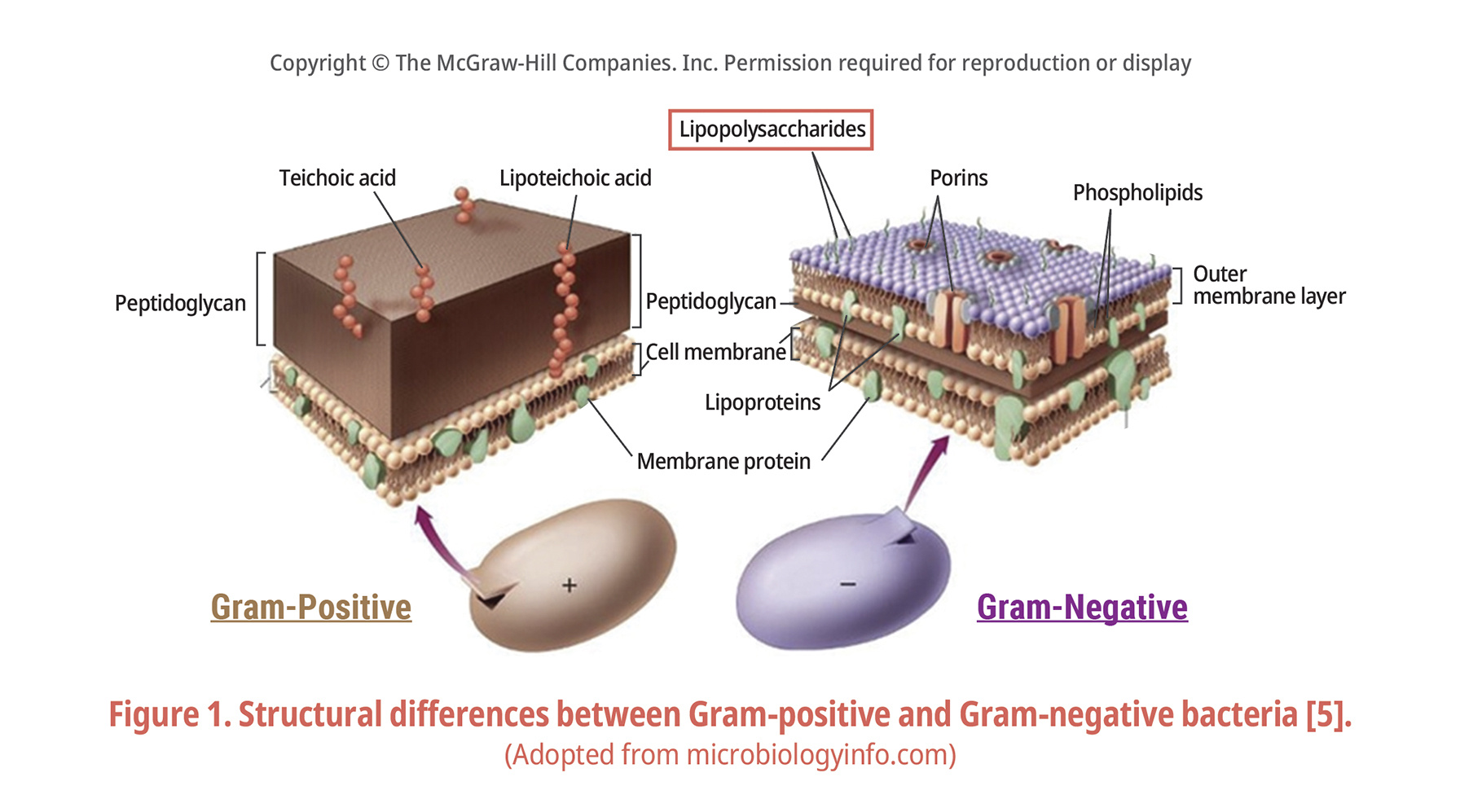
For both Gram-negative and Gram-positive bacteria, the cell wall consists of peptidoglycan (PG). However, in the case of Gram-negative bacteria, in addition to PG, there is an additional layer of lipopolysaccharide(LPS) composed of lipoprotein and protein, and this LPS layer has somatic antigen (O antigen) Lipid A, which has the characteristic of exhibiting toxicity (i.e., endotoxin) (Fig. 1). Due to the risk of these Gram-negative bacteria, companies in the pharmaceutical industry worry about the harmful effects such as pyrogenicity, lethality, Schwartzman reactivity [3], adjuvant activity and macrophage activation. It is essential to remove endotoxins from parenteral agents due to their biological activity [4].
Industrial use of microorganisms (amino acid production, etc.)
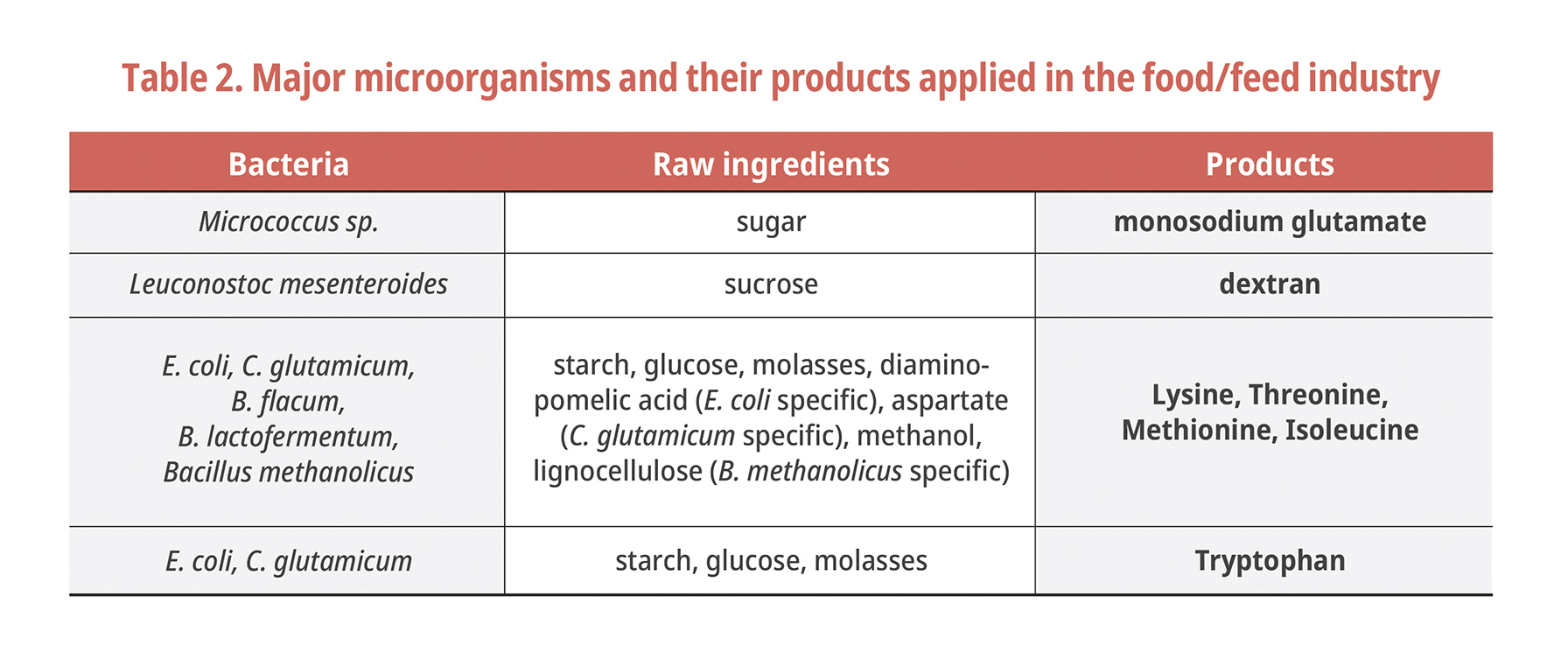
Mankind has been using microorganisms since 700 BC, and through antibiotics and biopolymers, it has recently been used in a variety of fields, from biofuels (fuel cells) to disease treatment (toxic element detection biosensors, cancer/ulcer disease elucidation). Both the Gram-negative bacteria and Gram-positive bacteria described above have important industrial usage. Among the traditional bulk products are ethanol, amino acids, organic acids, inorganic feeds, health foods, nutritional supplements, artificial sweeteners, and cosmetics (Table 2).
The amino acid market for feed additives has been developed along with the growth of the global livestock industry (because of an increased demand for vegetable protein sources and competition in feed costs). The volume and type of essential amino acids produced each year are increasing. (Demand for global L-lysine has been growing at CAGR of 14.7% during 2001-2015 [6]. China is the world's largest L-lysine producer, accounting for about 65% of the world's lysine production capacity [7,8]). The quantitative increase of amino acid production and the expansion of the product portfolio have been greatly improved by the fermentation and purification technology development and the strain improvement programs. The production of amino acids (L-glutamic acid) by bacteria was first produced in 1907 by Coryne. Thereafter, the development of efficient mutant strains for commercial production over the 1940-1950s increased economic viability with the mass production of various essential amino acids [9].
Utilization of killed bacteria as a feed additives
Harmful by-products are not found in almost all amino acid products. Rather, useful organic ingredients remain in the recovered by-products and are utilized as resources, such as feeder protein and fertilizer. However, the development of amino acid production technology for feeds such as granular form by simplifying (or omitting) the refinery process. This was an economical inevitability using bacteria from the fermentation incorporated into the final product in the form of killed/deactivated bacteria. Several studies have shown that killed lactic acid bacteria are stable to the environment due to strong resistance to acid and heat, are highly concentrated, easy to handle, and used as food for beneficial bacteria that have settled in the intestine, thereby enhancing the immune-enhancing activity unique to lactic acid bacteria [10].
However, it is unclear to date, whether the efficacy of these killed bacteria is the same for all strains. For example, comparative data is relatively small as to whether the observed efficacy in Gram-positive bacteria such as lactic acid bacteria is the same in Gram-negative bacteria having LPS known as endotoxin in the cell wall. Moreover, heat deactivation of Gram-negative bacteria promotes the release of LPS, and it is known that relatively heat-stable LPS still exists after heat treatment [4]. For example, the U.S. Pharmacopoeia (USP) recommends dry-heat treatment at temperatures above 220°C for as long as possible to reduce endotoxins to ≥3-log [11]. However, these temperatures have not been applied in general feed manufacturing processes. Therefore, the effect of feed containing killed bacteria as a protein or amino acid source in feed on the performance of animals may differ between Gram-negative and Gram-positive bacteria.
CONCLUSION
Currently, Corynebacterium glutamicum (Gram-positive) and Escherichia coli strains (Gram-negative) are most commonly used at an industrial level for the production of essential amino acids used in food, feed and pharmaceuticals. Therefore, as mentioned above, the recent market launch and expansion trend of feed additives as granular type amino acids and/or alternative protein sources containing killed/deactivated bacteria suggested that information on the type as strain (whether it is Gram-positive bacteria or Gram-negative bacteria) used as the raw additive is needed. Now, a variety of killed bacteria are on the market for alternative protein (amino acids) sources for feed, and it is time to make informed decisions based on accurate information on raw additive.
























































