Enriching eggs with DHA and its effects in human
2020년 09월 25일
- Nutrition
- Poultry
Abstract
Eggs are readily available and are considered as the cheapest source of protein with rich and balanced source of essential amino and fatty acids, minerals and vitamins, and is also now considered as an affordable source of docosahexaenoic acid (DHA) for the consumption of the general population without major cultural or religious prohibitions. Egg quality can be affected by certain nutrients in feed formulation. Enriching eggs with DHA increases its market value and profitability by selling it as a premium product. Consumption of sufficient amounts of omega-3 polyunsaturated fatty acids (PUFA) is associated with several health benefits for human. The n-3 PUFA (-linolenic acid (ALA), eicosapentaenoic acid (EPA), and DHA are essential nutrients that could not be produced sufficiently in the body. ALA can be found in plant oils. Although algae (micro- and macro-) and marine species such as in shellfishes, tuna, and salmon are rich in DHA and EPA, algal oil is considered as the most sustainable and eco-friendly source. A quantitative standard requirement for DHA is missing despite several studies showing positive DHA effects. DHA is an essential PUFA that plays a significant role in human brain, retina development, fetal development, nervous system development in early life, and reducing the risk of cardiovascular diseases in adults. DHA also serves as a structural component of membranes in the central nervous system, precursors of autacoid signaling molecules, and potent activators of several gene transcription. Thus, enrichment of meat and egg with DHA through their inclusion in animal feed is an important step towards healthy human nutrition.
Introduction
Diet plays a significant role in the survival and maintenance of health in animals and human. Eggs are readily available as an economic source of protein with rich and balanced profile of essential amino acids, fatty acids, minerals and vitamins (Khan, 2016; Omri et al., 2019; Surai et al., 2001). Eggs coming from laying hens fed with normal diets contain significant amounts of cholesterol (183 to 386 mg/egg) and saturated fatty acids (150 mg/egg) causing hypercholesterolemia thus encouraging a decline in egg consumption (Omri et al., 2019; Wang et al., 2017). Enriching eggs with n-3 PUFA is an important strategy to modify its lipid composition. The consumption of sufficient amounts of omega-3 fatty acids is associated with several benefits for human health. EPA (20:5, n-3), DHA (22:6, n-3) and ALA (18:3, n-3) are important n-3 PUFA in human nutrition (Aguillόn-Pez et al., 2020; Kalakuntla et al., 2017; Khan, 2016; Lee et al., 2019). ALA serves as a precursor for the synthesis of EPA and DHA with negligible efficiency. In human diet, the n-6 PUFA to n-3 PUFA ratio must be maintained at a balance ratio (4 to 1 or less), which could not be achieved with the typical modern human diets with a ratio of more than 10 to 1 (Kalakuntla et al., 2017). DHA is the focused essential fatty acid compared with the other n-3 PUFA because of its unique physicochemical characteristics and its structure that is highly unsaturated with its location mostly concentrated at the sn-2 position of phospholipids that forms the cell membrane, making it more fluid (Echeverria et al., 2017). Different technologies are applied to laying hens to further improve the quality and nutritional value of eggs in terms of antioxidants and n-3 PUFA (especially DHA) concentrations (Surai et al., 2001). DHA enriched eggs are possible by inclusion of ingredients high in DHA or n-3 PUFA in feed of laying hens.
Human DHA consumption is mostly derived from marine food, however, its accessibility to consumers becomes limiting due to price, availability, consistency, environmental concerns, and quality. Thus, considering DHA enriched eggs as an alternative source of DHA will provide one of the essential nutrients to human.
Sources of omega-3 fatty acids
Omega-3 fatty acids are essential nutrients that could not be produced sufficiently in the body but can easily be obtained from dietary sources like algae (micro- and macro-) and marine species such as shellfishes, tuna, salmon, etc. Marine-based ingredients typically contains high levels of EPA and DHA. EPA and DHA can also be produced endogenously by humans (Tocher et al., 2019), but the rate of biosynthesis is low and not enough to fulfill the physiological needs. Fishmeal and fish oil are the major sources of DHA in animal feed; though a high inclusion rate is required to provide a significant level of DHA for deposition either in the meat or in the eggs. High amount of fish oil in the animals diet may increase the risk of oxidation, sensory off-flavors (Aguillόn-Pez et al., 2020; Howe et al., 2020; Lee et al., 2019; Michalak et al., 2020; Moran et al., 2020; Omri et al., 2019; Wang et al., 2017), and the possible increase in the deposition of heavy metals originating from fish meal and fish oil.
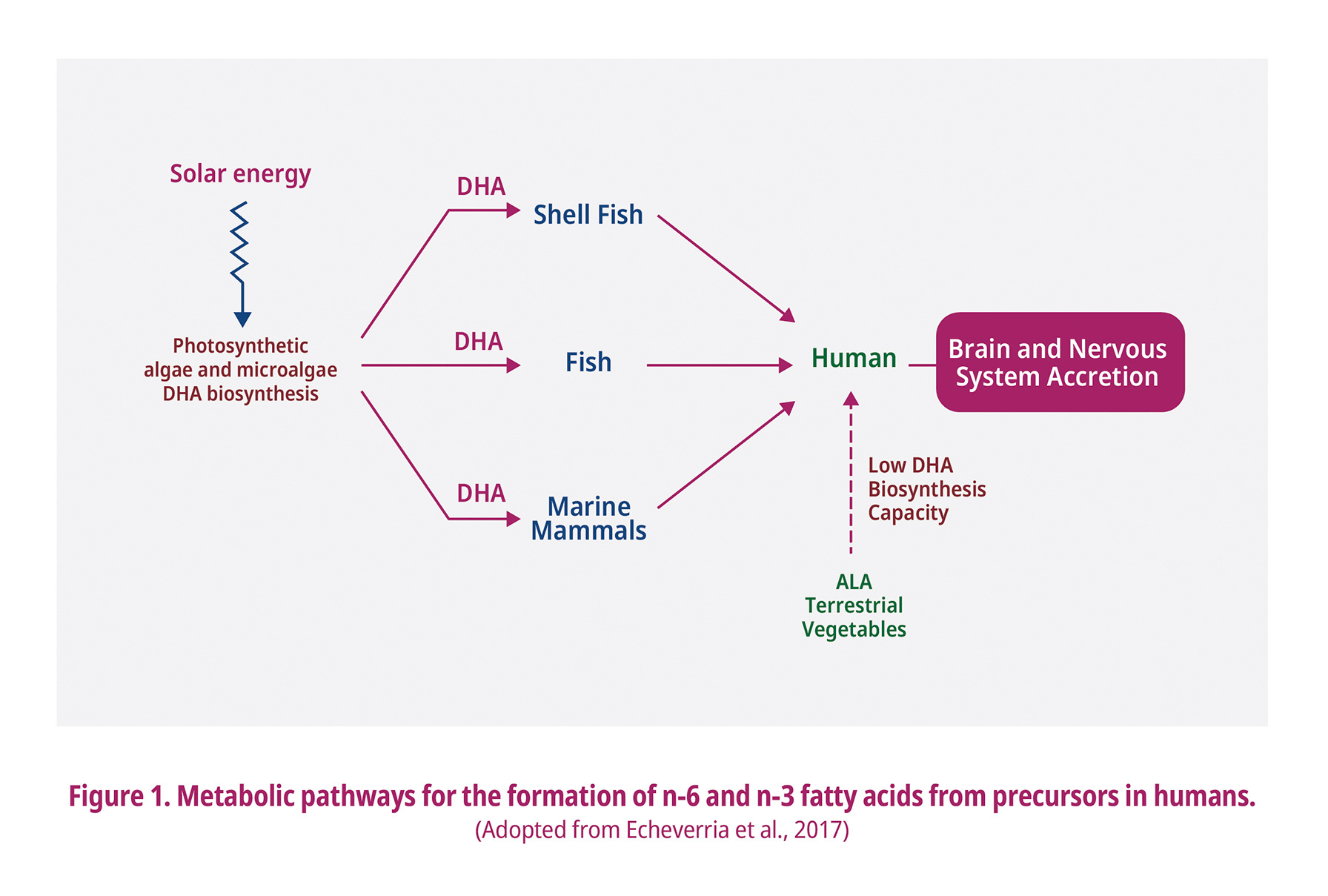
A high concentration of n-3 PUFA in egg yolk can also be achieved through a dietary supplementation of the laying hens feed with plant oils and algal oil. Oilseeds like canola, linseed, sunflower, full fat soya and camelina contains small amounts of DHA while having high amounts of ALA and their inclusion into broilers diet would increase the carcass ALA content without negatively affecting the sensory parameters (Kalakuntla et al., 2017). However, less than 1 to 4% of the consumed ALA is converted into DHA (Echeverria et al., 2017; Lauritzen et al., 2017; Lee et al., 2019; Moran et al., 2020), which occurs mainly in the liver (Fig. 1). Feeding laying hens with linseed (containing 17.27% of ALA) caused production of egg yolks which only possess 0.5% of EPA and 2.73% of DHA indicating to an inefficient conversion of ALA into EPA (Omri et al., 2019). Moreover, linseed in laying hens diet may also increase the susceptibility of egg lipids to oxidation. ALA is also one of the nutrients required by the commercial poultry breeder companies to fulfill the breeders ALA requirements as an essential nutrient.
Algae can also be a great source of n-3 PUFA, especially DHA. Global warming is predicted to reduce the de novo synthesis of DHA by algae (Colombo et al., 2020). Thus, human access to enough DHA has become limited. DHA deficiency may be significantly observed by the year 2100 because of climate change and overfishing thus can impact mostly the infants brain development (Colombo et al., 2020). Alternative sources of DHA will be beneficial to fulfill the requirement for this essential fatty acid. Furthermore, DHA is an essential long-chain PUFA that plays a significant role in human brain, retinal development, pregnancy, breastfeeding, early life nervous system development, and reducing the risk of cardiovascular diseases in adults (Dillon et al., 2019; Echeverria et al., 2017; Kaewsutas et al., 2017; Khan, 2016; Lee et al., 2019; Moran et al., 2018). DHA also serves as a structural component of membranes in the central nervous system, precursors of autocoid signaling molecules, and potent activators of several gene transcription factors (Ciappolino et al., 2019; Lauritzen et al., 2016).
Microalgae is also getting significant attention as a popular source of DHA through heterotrophic production and EPA by phototrophic production (Colombo et al., 2020; Lee et al., 2019; Torcher et al., 2019). Algae feeding to laying hens can significantly increase the EPA and DHA concentrations in eggs and can reduce the n-6 to n-3 ratio without negatively affecting the shelf-life and organoleptic properties of the products (Bruneel et al., 2013; Moran et al., 2018; Wang et al., 2017). However, the algal concentration of either DHA or EPA differs depending on the species of microalgae. The precise n-3 PUFA concentration of the microalgae biomass will influence its application in feeds (Tocher et al., 2019). The inclusion of supplemental DHA in the animal diets involves an extra cost, which is compensated with its positioning as premium product (Moran et al., 2020).
Benefits of DHA in poultry
The egg yolk is composed of 30% lipid with compositions depending on the age, strain and diet of laying hens (Aguillόn-Pez et al., 2020; Khan, 2016; Lee et al., 2019; Tocher et al., 2019). Inclusion of n-3 PUFA sources in laying hens diet increases the n-3 PUFA by about 7 to 12 times (Khan, 2016; Howe et al., 2002; Wang et al., 2017), which is about 220 mg per egg yolk that is equivalent to 100 g serving of lean fish. One n-3 PUFA enriched egg contains around 340 mg of ALA and 75 to 100 mg of DHA (Khan, 2016), while a 50 gram egg contains approximately 30 mg of DHA (Papanikolaou et al., 2018). As a result of increasing the source of n-3 PUFA in feed, the ratio of n-6/n-3 in egg decreases from around 15 towards a more balanced ratio of below 4 (Wang et al., 2017).
The meat of culled or spent laying hens may also be consumed, especially in some countries, and this meat is rich in protein, amino acids, PUFA and low in fat. Broilers fed diets supplemented with DHA originating from microalgae had an increase in the DHA content of meat (Moran et al., 2018). A reduction in the incidence of white striping has been related with increased levels of EPA and DHA and an increase in expression of glutathione peroxidase (an antioxidant in the poultry carcass) has been observed with an increased dietary PUFA (Lee et al., 2019; Moran et al., 2018).
The fatty acid composition of immune tissues is also highly related to the composition of dietary fat source (Lee et al., 2019). The inclusion of n-3 PUFA in diet improves cell flexibility and modulates functionality of specific immune cells. Moreover, supplementation of n-3 PUFA via microalgae in laying hens diet has the potential to reduce humerous bone breakage caused by poor bone strength and housing system (Lee et al., 2019).
Requirements for DHA and its effects in human
Most products in the market enriched with essential fatty acids are from poultry fed plant- and/or animal-based raw materials. There is no absolute quantitative standard requirement for DHA, despite the several studies that were published showing its positive effects. Though, Food and Agricultural Organization (FAO) recommended a minimum intake of DHA (200 mg/day) from a universal consensus (Echeverria et al., 2017). The international minimum recommendation of n-3 PUFA is 250 mg/day or 2 servings of oily fish for the general population (Aguillόn-Pez et al., 2020). In fact, the common EPA and DHA recommendation for cardiac health from over 50 organizations is 500 mg/person/day, while more than 1,000 mg of n-3 PUFA is consumed in a western-type diet according to the review of Tocher et al. (2019). Thus, the requirements for n-3 PUFA may depend on the purpose of supplementation. On the contrary, the daily recommended intake of EPA and DHA is 300 and 500 mg, respectively (Omri et al., 2019), because several factors are influencing the accessibility to DHA source such as income, lifestyle, geographical location, health status, etc.
The European Union recommends that food must contain at least 40 mg EPA + DHA per 100 grams in order to claim a n-3 fatty acid source, while 80 mg levels of EPA + DHA signifies that food is high in omega-3 (Moran et al., 2018). Considering eggs as a food option to supplement DHA will improve the accessibility of this essential fatty acids to most consumers. A study conducted in six-months old children fed with a formula containing egg yolk enriched with DHA (115 mg DHA/100 g food) and no maternal milk provision, showed a significant increase (34%) in DHA content of erythrocyte phospholipids and a better visual development at 12th month of age (Echeverria et al., 2017). One regular large egg (~50 grams) provides approximately 30 mg of DHA which is 30% of the 100 mg DHA recommended intake per day of the European Food Safety Authority for children between 6 and 24 months of age (Papanikolaou et al., 2018).
Pregnancy and child nutrition
Consumers became conscious on their health because of an increase in the incidence of non-infectious lifestyle diseases such as obesity, cardiovascular and degenerative diseases (Aguillόn-Pez et al., 2020; Kalakuntla et al., 2017; Khan, 2016). Thus, the market demands functional food to lower the risk of chronic diseases (Michalak et al., 2020).
A significant rise (23%) in EPA and DHA concentration in serum phospholipid is observed in statin-treated hypercholesterolemic male patients fed with specialty ration with 217 mg of DHA and 629 mg of total PUFA per day, which may be related to a lower risk for fatal ischemic heart disease (Gillingham et al., 2005). This is significant because there is a limited de novo synthesis of PUFA. DHA is necessary to be incorporated in the brain and tissues in the early life of infants and is dependent only to the maternal transfer from dietary supply through breastfeeding (Khan, 2016; Lauritzen et al., 2016). In addition, the DHA accumulation in the brain takes place from the intrauterine and neonatal period up to 2 years of age and the levels are maintained throughout life (Ciappolino et al., 2019; Lauritzen et al., 2016), yet, there are no studies on dietary requirements to meet the optimum DHA maintenance requirement of the brain.
In infants, cognitive and visual development is enhanced with dietary DHA as a major fatty acid in the phospholipids constructing the neuronal membranes and retina in the central and peripheral nervous system (Echeverria et al., 2017; Lauritzen et al., 2016; Smuts et al., 2003). Eggs enriched with DHA at around 135 mg DHA/egg as compared with ordinary eggs containing only 18 mg DHA/egg increases the DHA intake of mothers that would subsequently affect infants (Echeverria et al., 2017; Smuts et al., 2003).
DHA plays a significant role in neurogenesis and synaptogenesis especially in fetal development and during the first two years of life (Echeverria et al., 2017). There is an estimate of 50 mg/day calculation of whole-body DHA accretion during the third trimester of pregnancy (Lauritzen et al., 2016). Fetal DHA accretion happens actively during pregnancy, but it is most active during the third
trimester in pregnant women after the supplementation with fish oil at 200 mg DHA/day (Echeverria et al., 2017). Supplementation of DHA in pregnant women is not only beneficial to the fetus because of the efficient transport of fatty acids through the placenta, increase of milk DHA content, and the change in the composition of phospholipids in the umbilical cord blood during lactation. DHA is also important to limit the decline in maternal DHA status during the last trimester of gestation (Khan, 2016; Echeverria et al., 2017).
In toddlers, DHA-supplementation tends to promote a more relaxed mood in children and is effective in major psychiatric disorders like schizophrenia (Ciappolino et al., 2019; Echeverria et al., 2017; Lauritzen et al., 2016). Pre-scholar 4-year-old child supplemented with 400 mg DHA daily for 4 months showed significant higher blood DHA that was positively correlated with increment of punctuation obtained for vocabulary and comprehension tests (Echeverria et al., 2017).
Neuroprotection
DHA is an essential fatty acid for neuronal structure as well as signaling (Echeverria et al., 2017). DHA is identified as a neuroprotective agent against cerebral aging, neurodegenerative diseases, and cerebrovascular diseases. The proposed mechanism includes DHA maintaining the integrity and function of the neuronal membranes, preserves neuronal signaling pathways, and reduces neuronal death significantly (Ciappolino et al., 2019; Echeverria et al., 2017; Khan, 2016). Alzheimers disease, among the other neurodegenerative diseases showed the best proof of the benefits attributed to DHA at molecular level (Echeverria et al., 2017; Khan, 2016). Supplementation of 240 mg dietary DHA and 240 mg amino acids in older men diagnosed with cognitive impairment showed an improvement in the cognitive dysfunction associated to organic brain disease or to aging (Echeverria et al., 2017). DHA is also considered to participate in the preservation of cognitive skills during aging with its role in visual protection (Echeverria et al., 2017).
Conclusion
Eggs are an affordable source of highly bioavailable essential nutrients for the consumption of the general population. The nutritional value of eggs could be further enhanced with the enrichment of n-3 PUFA in the diets of laying hens impacting positively the health of a lot of people without the need for oral supplements. The positive effects of DHA in humans can compensate the premium price that DHA-enriched eggs may dictate in the market. Most importantly, the profitability of the producers will increase with the improvement of eggs market value. However, universal consensus on the daily recommended intake of n-3 PUFA, particularly DHA, must be determined.
























































